Research
Systematic confounder identification in the indication of relapsing-remitting multiple sclerosis (RRMS)
2023-2024
The Foundation for Quality and Efficiency in Health Care as the legal entity of the institute of the same name (IQWiG) has commissioned us to conduct a study on systematic confounder identification in the indication relapsing-remitting multiple sclerosis (RRMS) for the therapies with dimethyl fumarate and glatiramer acetate with regard to relapse rate, disability, quality of life and adverse events (GA23-02). The methodological approach follows Pufulete et al. (2022; https://doi.org/10.1016/j.jclinepi.2022.03.018).
CIEL-library
Cancer Immunotherapy Evidence – a Living Library
2023-2024
To explore, foster and promote the development and clinical implementation of novel immunotherapies such as therapeutic vaccines and adaptive cell therapies.
Perrine Janiaud (PI), Lars Hemkens; funded by Krebsliga beider Basel
Digital biomarkers
Since 2022
Through the development of digital health technologies in RC2NB’s work stream Digital Health, members of the Lab aim to develop approaches to remotely capture dynamic fluctuating functions and symptoms in real-world settings, which is key to novel pragmatic trial designs.
MultiSCRIPT
MultiSCRIPT: personalized medicine in MultipleSClerosis – pRagmatIc Platform Trial embedded within the Swiss MS Cohort (SMSC)
Since 2022
A learning trial platform that continuously evaluates novel personalized treatments for multiple sclerosis compared to usual care in a series of pragmatic trials. MultiSCRIPT aims to be a learning research and care system that rapidly integrates and continually evaluates innovative research findings in routine care.
Lars Hemkens (co-PI), Perrine Janiaud (Scientific coordinator); Investigator Initiated Clinical Trial (IICT) funded by Swiss National Foundation
PragMeta
Generalizability, applicability and pragmatism of clinical trials and their impact on treatment effect estimates: a meta-epidemiological study
Since 2019
PragMeta provides the first systematic large-scale empirical meta-epidemiological analysis of determinants of pragmatism and aims to reflect most recent conceptual developments and ensure that results optimally translate into future clinical research. More information on the PragMeta website and OSF.
Lars Hemkens (PI); funded by Swiss National Foundation
COVID-Evidence
COVID-evidence: a living database of trials on interventions for COVID-19 Swiss National Science Foundation
2019 to 2023
Continuously updated database of the worldwide available evidence on interventions for COVID-19, providing information on all planned, ongoing, and completed randomized controlled trials on any intervention to treat or prevent SARS-CoV-2-infections. We described the COVID-19 research agenda and highlight successes and lessons learned. More information on COVID-evidence website, OSF and GitHub.
Lars Hemkens (PI); funded by Swiss National Foundation
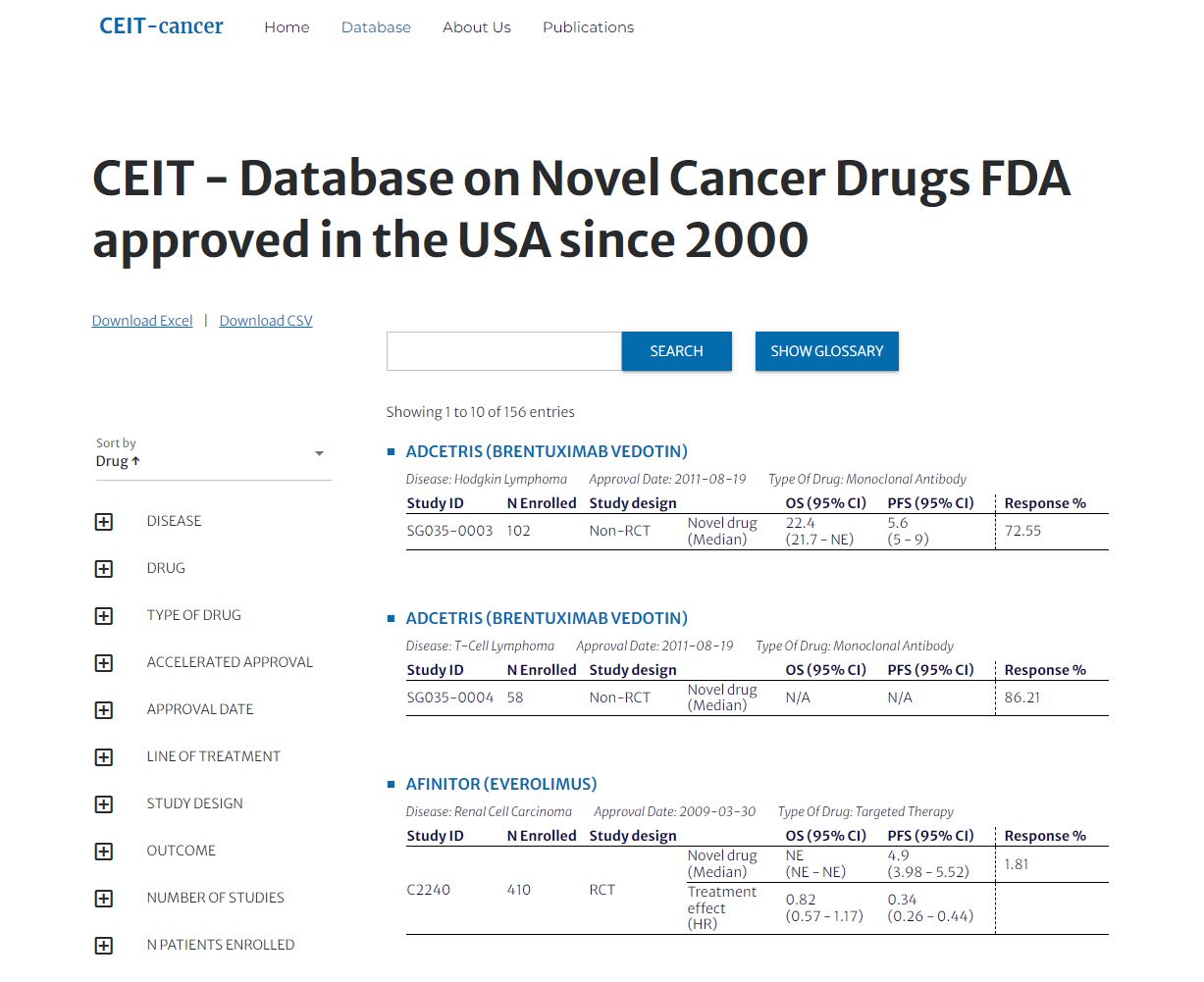
CEIT-Cancer
Comparative Effectiveness of Innovative Treatments for cancer
Since 2016
The Comparative Effectiveness of Innovative Treatments for cancer (CEIT-Cancer) project aims to provide a comprehensive and transparent overview of the early evidence supporting the approval of novel cancer therapies in the US. By collecting and assessing approval documents for all FDA-approved cancer drugs, the project hopes to improve evidence-based decision-making in policy and practice, and facilitate translation of the analyses into research and patient care. The project’s database is accessible through a dedicated website. More information on the CEIT-Cancer website.
Lars Hemkens (PI); funded by Swiss Cancer League
Collaborations
RECORD, RECORD-Pharmacoepidemiology, CONSORT ROUTINE
Transparency and reporting of research using routinely collected data (Real World Evidence)
Since 2013
Within a large worldwide network, we conducted numerous projects to improve the methods, transparency and reporting of research using routinely collected health data. This includes observational studies, comparative effectiveness research, and trials using routinely collected data and trials within cohorts and registries.
The RECORD-statement (REporting of studies Conducted using Observational Routinely-collected Data; RECORD) is the main reporting guidelines for observational studies conducted using routinely collected data.
CONSORT ROUTINE is an extension of the CONSORT-statement for reporting of randomized trials conducted using cohorts and routinely collected data .
This collaboration also includes the creation of a standardized protocol tool and eLearning program for research using routinely collected data.
(Co-Investigator: Lars Hemkens, funded by Canadian Institutes of Health Research)
Ethical issues in cluster randomized trials (Ottawa statement update)
Since 2022
The Ottawa Statement on the Ethical Design and Conduct of Cluster Randomized Trials aims to provide detailed guidance on the ethical design, conduct, and review of Cluster Randomized Trials. In this project, an update of this guidance will be developed using stakeholder and patient engagement to generate guidance for the ethical design and conduct of trials evaluating clinical, health policy, health systems, and public health interventions.
(Co-Investigator: Lars Hemkens, funded by Canadian Institutes of Health Research)
RefHunter – Systematic literature searching
Since 2017
Within a trinational network, RefHunter provides a platform for systematic literature searching; specifically, aiming to systematically characterize health-related electronical databases and to guide the development of database-specific search strings, and, ultimately, to improve the quality of literature searches in health research.
(Co-Investigator: Julian Hirt, platform infrastructure funded by Network for Evidence-Based-Medicine)
Partners, Networks, and Institutions
In addition to numerous collaborations with research groups at the University of Basel, we are working with a variety of partners in Switzerland and worldwide.
Close and continuous collaborations are with
- Meta-Research Innovation Center at Stanford (METRICS), Stanford University, USA
- Meta-Research Innovation Center Berlin (METRIC-B), Berlin Institute of Health / Charité and Max Delbrück Center, Berlin, Germany
- QUEST Center for Responsible Research, Berlin Institute of Health / Charité and Max Delbrück Center, Berlin, Germany
- CEIR – Centre for Epidemic Interventions Research, Norwegian Institute of Public Health, Oslo, Norway
- Institute of Health and Nursing Sciences, Medical Faculty, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany
- Department of Health, Eastern University of Applied Sciences, St.Gallen, Switzerland
- Center for Health Services Research (ZVF-BB), Brandenburg Medical School Theodor Fontane, Rüdersdorf, Germany
- GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group via McMaster University, Canada
- Evidence-based Medicine Research Group at University Hospital Cologne, Cologne, Germany
- High Impact Events Preparedness unit (IEP) at World Health Organization, Geneva, Switzerland